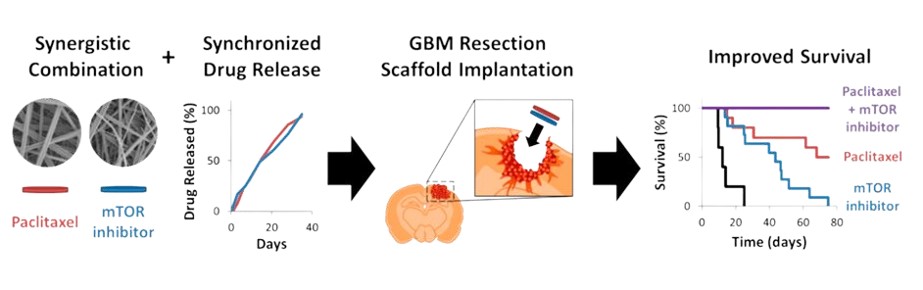
In the early 1990s, a significant breakthrough emerged in the challenging landscape of aggressive brain tumor treatment, particularly for conditions like glioblastoma multiforme (GBM). This advancement, the Gliadel Wafer, was not merely a new therapeutic agent but a testament to the transformative power of interdisciplinary innovation, particularly at the intersection of materials science and neuro-oncology.The formidable challenge in treating brain tumors lies in the brain's inherent protective mechanism: the blood-brain barrier(BBB).This highly selective physiological barrier, while crucial for safeguarding the central nervous system simultaneously impedes the delivery of most chemotherapeutic agents to the tumor site.
Traditional approaches often grappled with this impermeability, necessitating methods such as osmotic disruption of the BBB or direct intrathecal injections via devices like Ommaya reservoirs, each with their own complexities and limitations in terms of precision and systemic exposure.
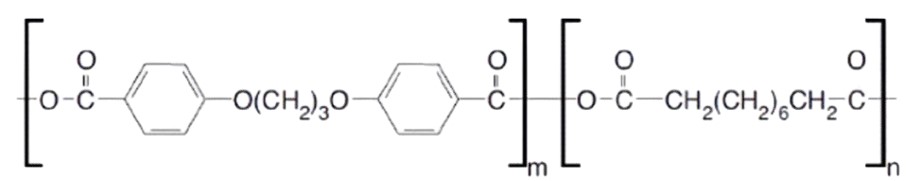
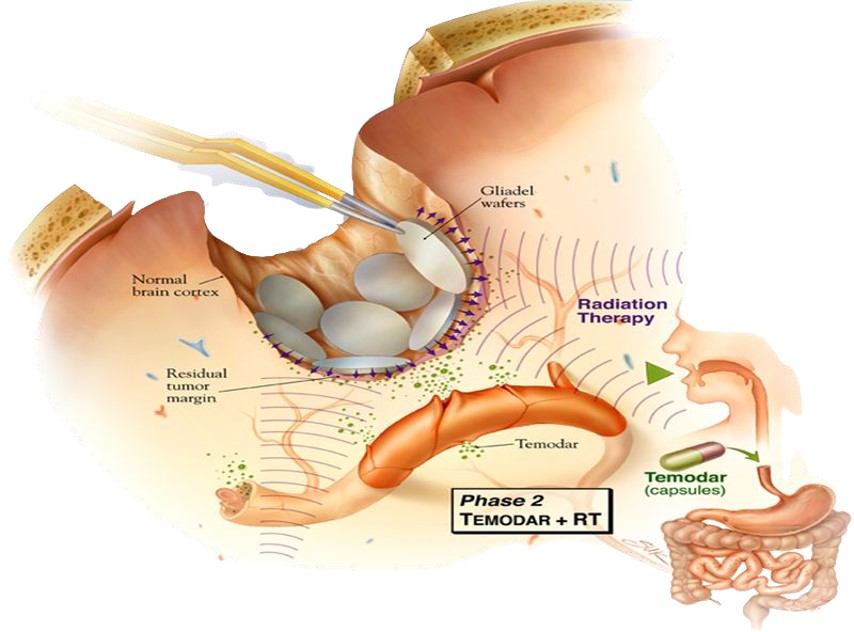
The Gliadel Wafer, however, presented an elegant solution. This biodegradable implant, approximately 1.45 cm in diameter and 1 mm thick, was meticulously engineered from a polyanhydride copolymer, specifically polifeprosan 20 (a 20:80 molar ratio of sebacic acid and poly[bis(p-carboxyphenoxy)]propane). Crucially, this polymer matrix uniformly encapsulated 7.7 mg of carmustine (BCNU), a potent alkylating agent.
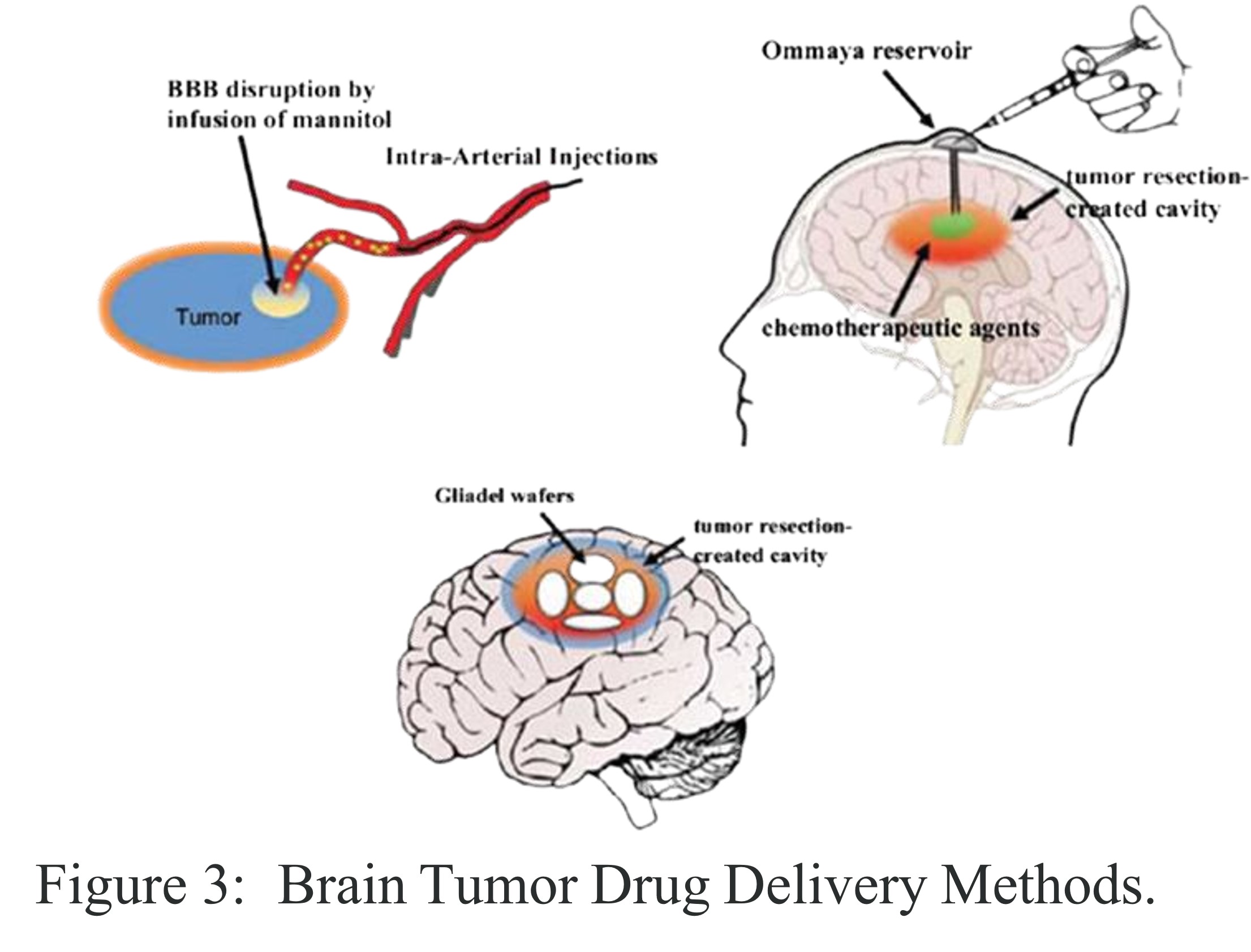
Following the surgical removal of the brain tumor, sterile wafers, varying in color from off white to pale yellow, were carefully placed into the resection cavity. The innovation of Gliadel lay in its ability to deliver drugs locally and in a sustained manner. As the biodegradable polymer matrix gradually degraded, it released carmustine directly into the surrounding brain tissue, avoiding entry into the systemic circulation. This mechanism provided significant benefits by delivering a concentrated dose of carmustine directly at the tumor site. As a result, Gliadel reduced systemic toxicity, lowering the risks of organ damage and immunosuppression that are often associated with intravenous chemotherapy. Furthermore, this localized delivery approach demonstrated improved patient survival outcomes, especially in cases of recurrent brain tumors.
The Giladel Wafer exemplifies how a deep understanding of polymer chemistry and material degradation kinetics can be harnessed to address complex biomedical challenges. The design of polifeprosan 20, with its controlled release characteristics, was not serendipitous but the result of meticulous research into optimizing polymer composition for therapeutic efficacy.The challenges of medicine are often solvable through the innovation of materials. The Gliadel Wafer is a testament to how designing materials at the molecular level can have macroscopic, life-altering consequences.Understanding of structure-property relationships, the ability to synthesize novel compounds, and creativity in engineering solutions will be the bedrock upon which the next generation of medical breakthroughs are built. Therefore, may it be remembered that the profound potential to heal, to extend life, and to elevate human well-being frequently finds its core within a material.