
Sustainable efforts are increasingly relying on intelligent, biodegradable polymers. These materials are designed to respond to changes in heat, light, or moisture, almost as if they possess intelligence. When made from materials like PLA (polylactic acid) or PCL (polycaprolactone), they can even change their shape, color, or substance in the right environment. This breakthrough has paved the way for eco-friendly packaging, dissolvable sutures, and slow-acting agricultural films, and could even lead to self-healing smartphone screens that repair themselves after a crack.
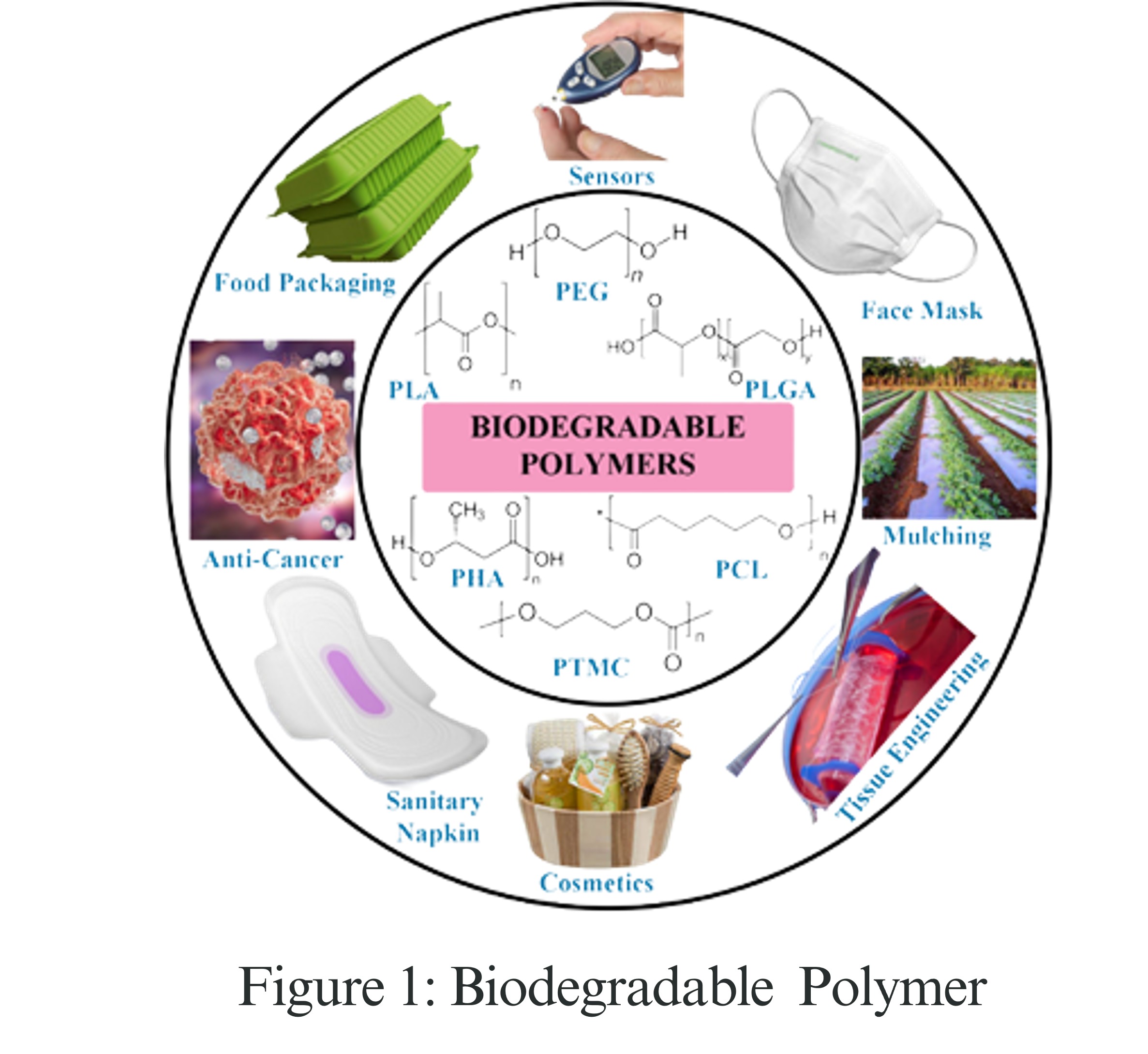
How Do These Materials React?
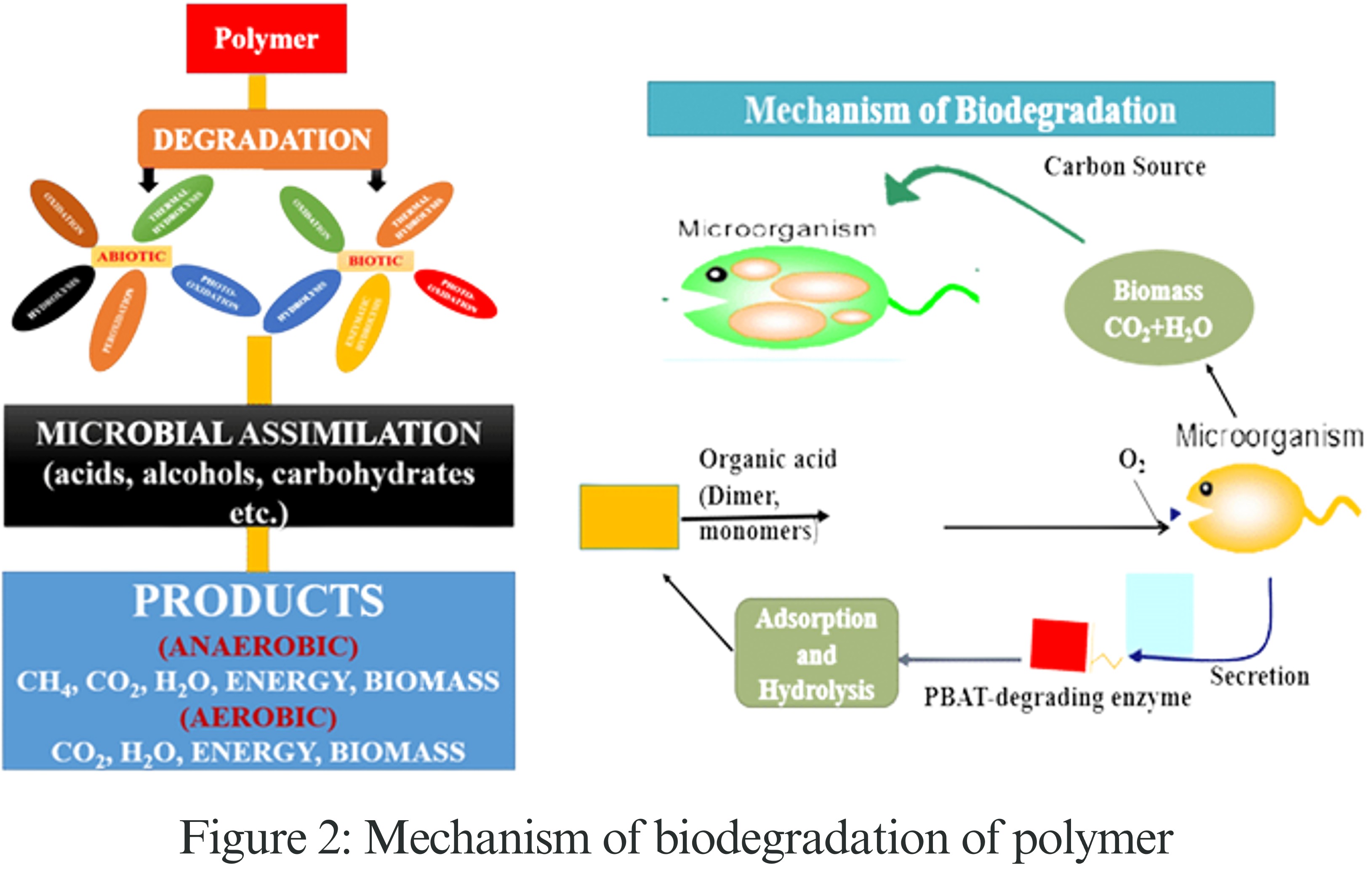
How Biodegradable Polymers Break Down: Naturally, tiny living things like bacteria and fungi eat away at biodegradable plastics over time. First, things in the environment like sunlight or moisture damage the surface of the plastic.
Then, these tiny living things release special chemicals called enzymes that cut the long plastic chains into much smaller pieces. This process is called depolymerization. After these smaller pieces are formed, the tiny living things take them in and use them for energy and food. Finally, through a process called mineralization, the plastic breaks down into common substances, including carbon dioxide (CO₂), water (H₂O), methane (CH₄), and biomass. For example, PLA (polylactic acid) turns into lactic acid and is then broken down by microbes into CO₂ and water, which is safe for the environment.
How Smart Polymers Repair Themselves: Smart polymers can fix themselves without outside help because of their special chemistry. Many scientists have studied a process called the Diels–Alder reaction, where certain parts, like furan and maleimide, can form and break bonds easily.
When something breaks the plastic, these bonds break, but with a little heat (60–120°C), they can reattach and fix the damage. Hydrogen bonding is another way these plastics work; their chains line up and connect again with very weak, easily breakable bonds. Also, some plastics have disulfide bonds that can break and form again under certain conditions, allowing these materials to repair themselves.
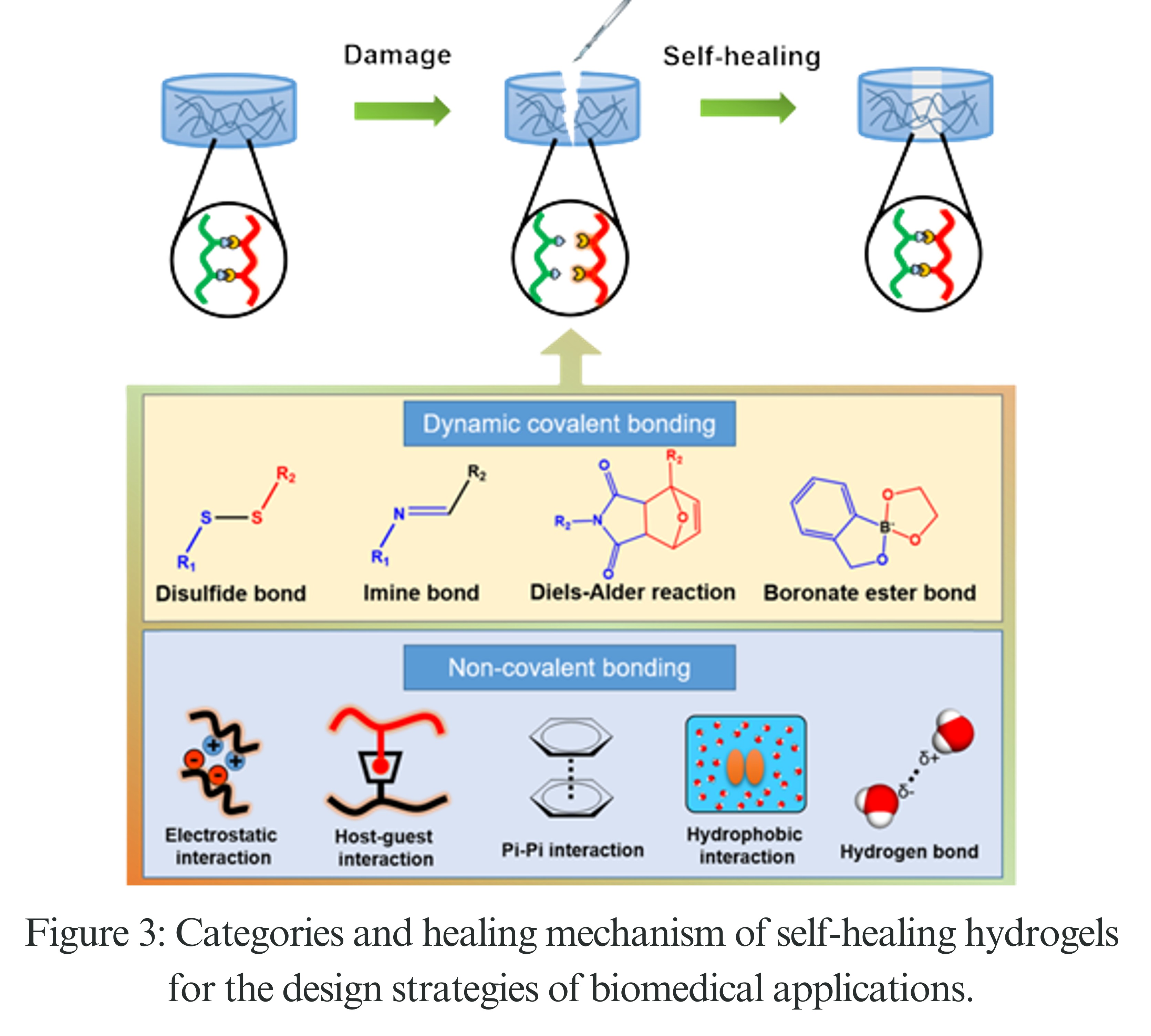
Let this exploration of smart, self-healing, and vanishing materials ignite a passion within materials science and engineering students and researchers, driving us to innovate solutions for a truly sustainable tomorrow.